Spinraza

Nusinersen, marketed under the brand name Spinraza, stands as the first-ever FDA-approved treatment for Spinal Muscular Atrophy (SMA). Administered through intrathecal injections directly into the cerebrospinal fluid, this groundbreaking medication utilizes “antisense oligonucleotides” (ASOs) to modify gene expression, targeting the affected motor neurons in the spinal cord and brain.
Clinical trials have showcased the remarkable effectiveness of nusinersen in enhancing motor function, achieving significant milestones, and increasing survival rates for individuals with SMA. With encouraging results across various types and stages of the disease, nusinersen instills hope and optimism for patients and their families.
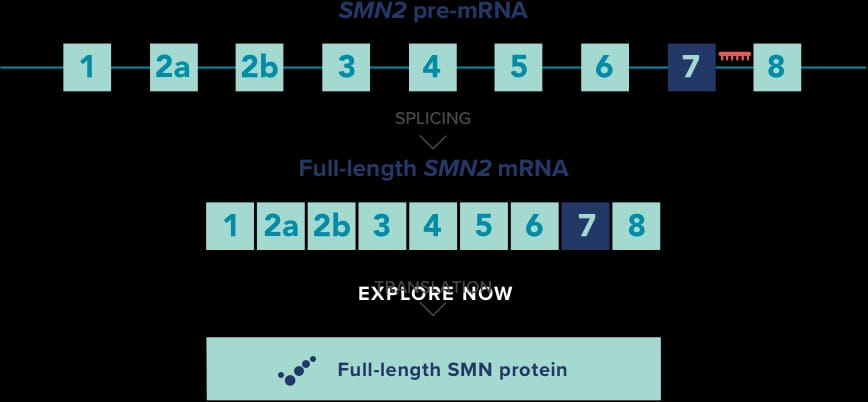
Moreover, nusinersen has demonstrated its potential to slow disease progression, thereby reducing the need for respiratory support and hospitalizations in certain cases. This transformative treatment has become a pivotal alternative to previously available supportive care, significantly altering the management of SMA and positively impacting those affected by this challenging neuromuscular disorder.
Ongoing research and clinical trials persist in exploring the long-term effects of nusinersen and potential combinations with other therapies for the best possible outcomes. Since its approval, nusinersen has stimulated increased interest and investment in research for other promising SMA treatments and therapies. Its success has paved the way for further advancements, including ASO-based treatments and gene therapies targeting SMA and related neuromuscular conditions.
Beyond its impact on patients and families, nusinersen’s approval has initiated critical discussions on drug pricing and accessibility. As a groundbreaking and life-changing treatment, ensuring affordability and equitable access has become an essential topic in the healthcare community.
Overall, nusinersen represents a remarkable achievement in medical science and a significant breakthrough in the treatment of SMA. As research continues and new discoveries unfold, the hope is that this progress will extend to other rare genetic diseases, unlocking new possibilities and opportunities for patients worldwide.
Overall, nusinersen represents a remarkable achievement in medical science and a significant breakthrough in the treatment of SMA. As research continues and new discoveries unfold, the hope is that this progress will extend to other rare genetic diseases, unlocking new possibilities and opportunities for patients worldwide.
To access the complete details, follow the provided link.
