Zolgensma

Zolgensma, also known by its generic name onasemnogene abeparvovec-xioi, is a gene therapy treatment approved by the FDA for the treatment of Spinal Muscular Atrophy (SMA). Developed by AveXis, a Novartis company, Zolgensma represents a significant breakthrough in the management of SMA. It was granted FDA approval on May 24, 2019.
Zolgensma works by addressing the underlying genetic cause of SMA, which is a deficiency in the survival motor neuron 1 (SMN1) gene. It utilizes a single-dose intravenous infusion to deliver a functional copy of the SMN1 gene to the patient’s cells. The treatment employs a viral vector, specifically an adeno-associated virus (AAV), to deliver the corrected gene into the motor neuron cells. Once inside the cells, the functional SMN1 gene produces the crucial survival motor neuron (SMN) protein that is lacking in individuals with SMA.
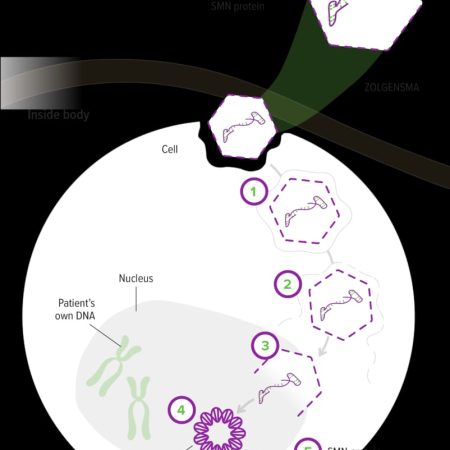
By restoring the production of the SMN protein, Zolgensma aims to halt or slow down the progression of SMA and improve motor neuron function. It has demonstrated remarkable clinical benefits, including improved motor function, increased survival, and developmental milestones achieved in infants and young children with SMA.
Zolgensma, also known as onasemnogene abeparvovec-xioi, is considered a transformative therapy due to its potential to provide long-lasting effects with a single administration. However, it is important to note that the therapy is most effective when administered early in the course of the disease before irreversible motor neuron loss has occurred.
The approval of Zolgensma has brought new hope to individuals and families affected by SMA, offering a promising treatment option to address the underlying genetic cause of the disease and potentially improve the quality of life for patients with SMA.
To access the complete details, follow the provided link.
